The push towards targeted cancer therapy has established gene fusions as a targetable class of genetic mutations. Targeted treatment has generated survival rates far higher than those achieved with standard chemotherapy. NTRK gene fusions are one such targetable aberration.
The NTRK Gene Family
The neurotrophin receptor tyrosine kinase (NTRK) family is made up of 3 genes crucial to nervous system development and physiology. These genes - NTRK1, NTRK2, and NTRK3 - encode the receptors TRKA, TRKB, and TRKC, respectively. Each receptor is activated by a different binding factor, which triggers phosphorylation of the proteins’ intracellular kinase domain, setting off various signaling pathways that maintain normal nervous system function.1
NTRK1
NTRK1 is found on chromosome 1q21-q22. The gene is expressed in specialized neurons of the basal forebrain that monitor memory processes, pain, and temperature sensing.1 NTRK1’s encoded protein, TRKA, contains an intercellular domain containing a juxtamembrane region, a TK domain, and a short C terminal tail. TRKA serves as a receptor for nerve growth factor beta (NGFb). Upon binding with NGFb, TRKA phosphorylates itself and other MAPK pathway proteins.
NTRK2
NTRK2 maps to chromosome 9q22.1. The gene encodes the TRKB receptor, which binds brain-derived neutrotrophic factor (BDNF) and neutrophin-4 (NTF4). TRKB expression is highest in the prefrontal cortex, amygdala, and occipital lobe.1,2 The gene promotes neural cell plasticity and survival, as well as synaptic signaling and possibly even mood stabilization. TRKB also assists in learning and memory by aiding both short term synaptic function and long-term potentiation.5
NTRK3
NTRK3 maps to chromosome 15q25. Its encoded protein, TRKC, is a glycoprotein primarily expressed in the hippocampus, cerebral cortex and the granular cell layer of the cerebellum. 9 NTRK3 mediates neuronal differentiation and survival via activation of the MAPK and PI3K/AKT pathways. The gene may also aid in proprioception (via differentiation of body position-sensing neurons) and heart development (TrkC null mice nearly always die within the first weeks of life from severe cardiac defects).1,2,3,4
A Brief History of NTRK in Cancer
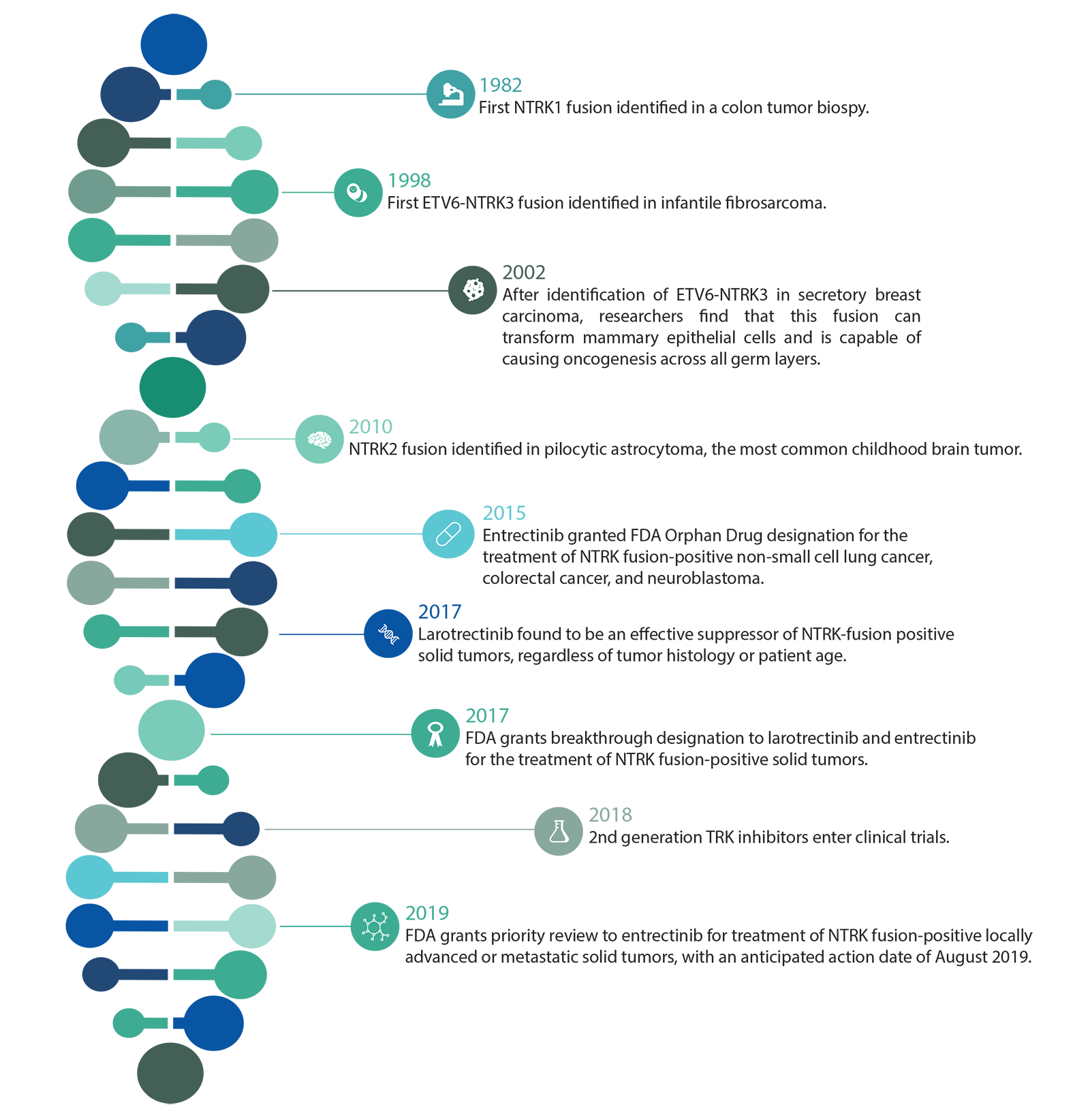
How Gene Fusions Drive Cancer
Gene fusions occur when two normally separated genes are joined, which can contribute to cancer development in a few different ways:
A gene may become overexpressed when it fuses to a chromosomal location with unfamiliar promoters.5
Two genes can fuse to create a new hybrid gene encoding an oncogenic protein.5

NTRK genes almost always become oncogenic through this mechanism:


Fusions can lead to truncation and resulting inactivation of important tumor suppressor genes.5
NTRK's 3’ region fuses with the 5’ sequence of a partner gene, creating a chimeric fusion protein with a constitutively activated NTRK kinase domain. This leads to deregulated neural development – neuron growth, differentiation, proliferation, and apoptosis all fall into disarray, and important synaptic pathways are compromised.5
How Gene Fusions Drive Cancer
Gene fusions occur when two normally separated genes are joined, which can contribute to cancer development in a few different ways:

A gene may become overexpressed when it fuses to a chromosomal location with unfamiliar promoters.5

Fusions can lead to truncation and resulting inactivation of important tumor suppressor genes.5

Two genes can fuse to create a new hybrid gene encoding an oncogenic protein.5

NTRK genes almost always become oncogenic through this mechanism:

NTRK's 3’ region fuses with the 5’ sequence of a partner gene, creating a chimeric fusion protein with a constitutively activated NTRK kinase domain. This leads to deregulated neural development – neuron growth, differentiation, proliferation, and apoptosis all fall into disarray, and important synaptic pathways are compromised.5
NTRK Fusion Partners
NTRK fusions are highly promiscuous; so far, over 80 different 5’ fusion partners have been discovered, including those listed below. NTRK1 partners are mostly found on chromosome 1, indicating that intrachromosomal rearrangements are the main drivers of NTRK1 fusions. NTRK2 and NTRK3 fusions, on the other hand, usually occur via interchromosomal rearrangements.8
| NTRK GENE | FUSION PARTNER | ASSOCIATED CANCERS |
|---|---|---|
| NTRK1 | IRF2BP2 | Lung cancer, thyroid cancer, prostate cancer |
| LMNA | Appendiceal cancer, breast cancer, cholangiocarcinoma, colorectal cancer, gallbladder carcinoma, soft tissue sarcoma, Spitzoid neoplasm, uterine sarcoma | |
| TPM3 | Breast cancer, cervical cancer, cholangiocarcinoma, colorectal cancer, glioma, infantile fibrosarcoma, lung cancer, soft tissue sarcoma, thyroid cancer, uterine sarcoma | |
| TPR | Lung cancer, thyroid cancer, uterine sarcoma, pediatric mesenchymal tumor | |
| NTRK2 | DAP21P | Colorectal cancer |
| NOS1AP | Anaplastic astrocytoma, glioma | |
| SQSTM1 | Glioma, lung cancer | |
| TRAF2 | Melanoma | |
| NTRK3 | EML4 | Congenital mesoblastic nephroma, glioma, infantile fibrosarcoma, thyroid cancer |
| ETV6 | Acute lymphoblastic leukemia, acute myeloid leukemia, breast cancer, colorectal cancer, congenital mesoblastic nephroma, gastrointestinal tract stromal tumor, glioma, infantile fibrosarcoma, inflammatory myofibroblastic tumor, lung cancer, melanoma, neuroendocrine cancer, secretory breast cancer, secretory carcinoma of salivary gland, sinonasal adenocarcinoma, soft tissue sarcoma, Spitzoid neoplasm, thyroid cancer | |
| RBPMS | Thyroid cancer, uterine sarcoma |
NTRK Fusion Prevalence in Human Cancers
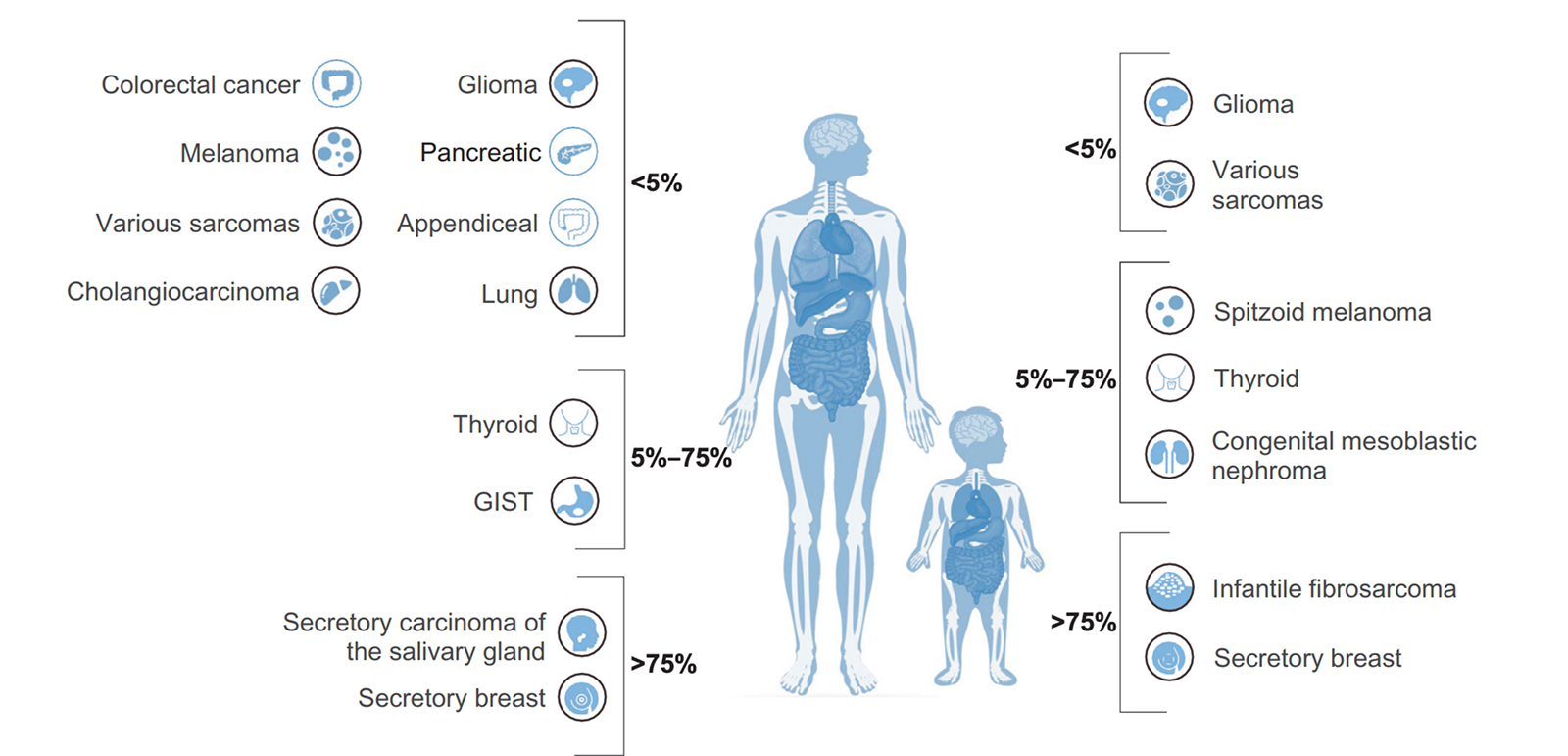
Recent studies have shown that NTRK fusions contribute to tumorigenesis in over 90% of infantile fibrosarcoma, congenital mesoblastic nephroma, and breast secretory carcinoma.6 Because of their high prevalence in these rare, poorly understood and highly aggressive cancers, NTRK fusions are sometimes the only actionable targets for gene-specific treatment. They also occur with lower frequency in an additional 16 common malignancies, including colorectal cancer, thyroid cancer and non-small cell lung cancer.6 Additionally, NTRK fusions are the only oncogenic abnormality present in 29% of NTRK fused cancers, further evidence of the gene family’s utility in diagnosis.7 NTRK aberrations have also been implicated in certain neuronal disorders, including anhidrosis, epilepsy and depression.5
Cancers with the highest prevalence of NTRK fusions
Infantile Fibrosarcoma
Infantile Fibrosarcoma (IF) is an extremely rare childhood tumor, but is the most common nonrhabdomyosarcoma soft tissue tumor seen in infants < 1 year old. ETV6-NTRK3 fusions have been shown to occur with between 70% and 91% frequency in these tumors, while other pediatric spindle cell neoplasms lack the fusion. LMN1-NTRK1 and EML4-NTRK3 fusions have also been detected in IF.
Secretory Breast and Salivary Gland Carcinomas
Secretory Breast Carcinoma (SBC) is a rare subset of infiltrating ductal carcinoma that harbors ETV6-NTRK3 fusions with over 90% frequency. Recently, a similar tumor type was also described in the salivary gland, which morphologically and immunohistochemically resembled SBC. These salivary gland carcinomas also display ETV6-NTRK3 fusions in more than 90% of cases. ETV6-NTRK3 fusions have also been detected in secretory carcinomas of the skin and thyroid.
Congenital Mesoblastic Nephroma
This rare tumor occurs in the spindle cells of the kidney, most commonly in newborns or young infants. CMN has two three histologic subytpes – classic, cellular and mixed, with the latter two being far more rare. In nearly all cases of cellular and mixed CMN, ETV6-NTRK3 fusions are found, but haven’t yet been detected in classic CMN. The fusion usually occurs alongside trisomy 11.
Thyroid Carcinoma
Thyroid cancers in patients younger than 20 years of age account for more than 2% of all thyroid malignancies diagnosed in the US. Of these diagnoses, papillary thyroid carcinoma (PTC) makes up about 90%. In one study of 27 pediatric PTC patients, NTRK fusions were found in 26% of cases. NTRK fusions are also found in adult thyroid cancer patients, although at a lower frequency (<10%).
Brain Tumors
In a study of 112 pediatric high-grade gliomas, NTRK fusions were detected in 8 of the patients, and were more common in non-brainstem high-grade glioma in patients < 3 years.
Other cancers known to harbor NTRK fusions:
NTRK fusions have also been detected, albeit in lower frequencies, in a variety of common cancers, including:
- Breast cancer
- Non-small cell lung cancer
- Colorectal Cancer
- Melanoma
Generally, NTRK fusion frequency doesn’t exceed 5%. However, as widespread screening for NTRK becomes more common, incidence of these fusions will likely be better reported. In the coming years, therefore, we’ll achieve a more accurate idea of the true prevalence of NTRK fusions across the major cancer types.8
References:
1. Amatu A, et al. (2016) ESMO open 1.2: e000023.
2. Raez LE, et al. (2016) Future Medicine: 5.1: 1-4.
3. NIH.gov
4. UniProt.org
5. Mertens F, et al. (2015). Nat Rev Cancer 15.6: 371–381.
6. Cocco Emiliano, et al. (2018) Nat Rev Clin Onc: 1.
7. Gatalica, Zoran, et al. (2019) Mod Pathol 32: 147-153.
8. Hsiao, Susan J., et al. (2019) Jour Molec Diag.
9. Genecards.org